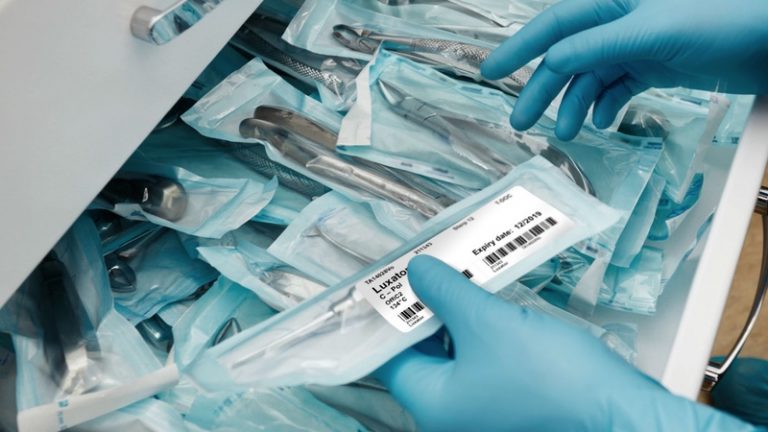
The European Union (EU) Medical Device Regulation (MDR), implemented on May 26, 2021, mandates specific requirements regarding the labeling of medical devices. Medical device manufacturers must comply with these new […]
Tuesday, Apr 16, 2024
Breaking News